| TOXICITÉ DES TRAITEMENTS |
| Chimiothérapies et grossesse : quelles doses ? |
Abstract![]()
Background: Pregnant patients with cancer are increasingly treated with anti-cancer drugs, although the specific impact of pregnancy-induced physiological changes on the pharmacokinetics (PK) of anti-cancer drugs and associated implications for optimal dose regimens remains unclear. Our objectives were to quantify changes in PK during pregnancy for four frequently used anti-cancer agents doxorubicin, epirubicin, docetaxel and paclitaxel, and to determine associated necessary dose adjustments.
Patients and methods: A pooled analysis of PK data was performed for pregnant (Pr) and non-pregnant (NPr) patients for doxorubicin (n = 16 Pr/59 NPr), epirubicin (n = 14 Pr/57 NPr), docetaxel (n = 3 Pr/32 NPr) and paclitaxel (n = 5 Pr/105 NPr). Compartmental nonlinear mixed effect models were used to describe the PK and gestational effects. Subsequently we derived optimized dose regimens aiming to match to the area-under-the-concentration-time-curve (AUC) in non-pregnant patients.
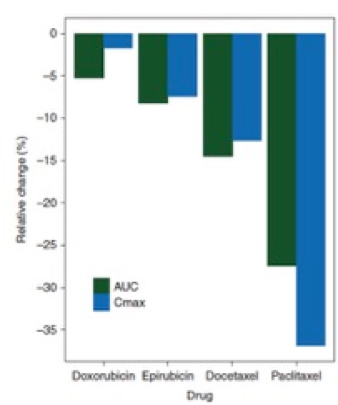
Results: The effect of pregnancy on volumes of distribution for doxorubicin, epirubicin, docetaxel and paclitaxel were estimated as fold-change of <1.32, <2.08, <1.37 and <4.21 respectively, with adequate precision (relative standard error [RSE] <37%). For doxorubicin, no gestational effect could be estimated on clearance. For epirubicin, docetaxel and paclitaxel a fold-change of 1.1 (RSE 9%), 1.19 (RSE 7%) and 1.92 (RSE 21%) were respectively estimated on clearance. Calculated dose adjustment-requirements for doxorubicin, epirubicin, docetaxel and paclitaxel were +5.5%, +8.0%, +16.9% and +37.8%, respectively. Estimated changes in infusion duration were marginal (<4.2%) except for paclitaxel (−21.4%).
Conclusion: Clinicians should be aware of a decrease in drug exposure during pregnancy and should not a priori reduce dose. The decrease in exposure was most apparent for docetaxel and paclitaxel which is supported by known physiological changes during pregnancy. The suggested dose adaptations should only be implemented after conduct of further confirmatory studies of the PK during pregnancy.
Keywords: pharmacokinetics; pregnancy; modeling; anti-cancer drugs; chemotherapeutics;
Références de l'article original :
![]()
J. G. C. van Hasselt, K. van Calsteren, L. Heyns, S. Han, M. Mhallem Gziri, J. H. M. Schellens, J. H. Beijnen, A. D. R. Huitema and F. Amant - Annals of Oncology 2014; 25(10): 2059-65
| Cliquez pour lire la synthèse |